Medical Injection Molding Case Study: Blood Glucose Meter Panel Production
2025/10/10 By le zhan

Many medical device recalls stem from component failures, which can often be traced back to inaccurate injection molding processes. For a client I work with that produces blood glucose meter panels, this risk is particularly acute. A dimensional deviation of even 0.1 mm can cause internal sensors to misalign, leading to inaccurate blood glucose readings or even device failure. These defects are not only costly for manufacturers but also undermine the trust of healthcare providers and patients. Therefore, we will use this blood glucose meter panel case study as a focal point to illustrate how all-electric injection molding machines address the pain points of medical injection molding production: inconsistent part dimensions, long cycle times, and insufficient regulatory compliance.
Case video: https://www.youtube.com/watch?v=QNNgr6pULtc&t=1s
Medical Injection Molding Project Overview: Blood Glucose Meter Panel Specifications and Requirements
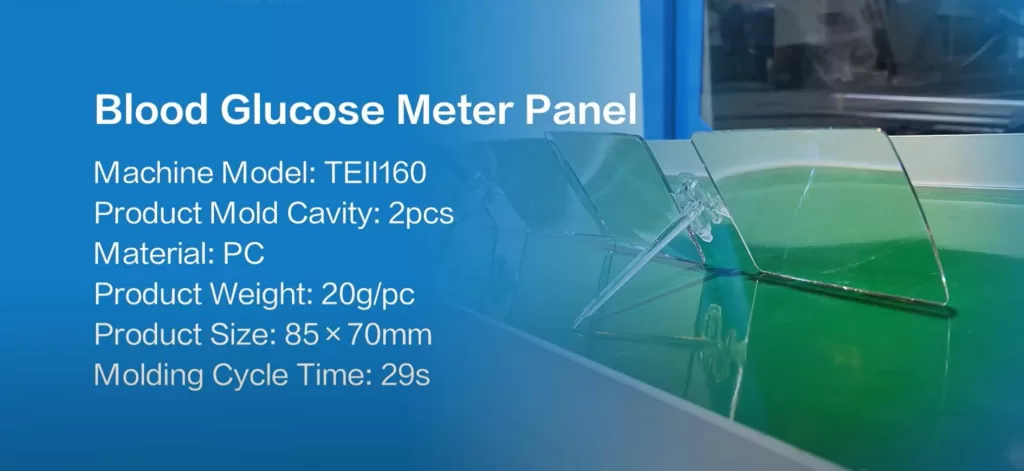
Let’s first review the key parameters of this project. The client, a medical device manufacturer, needed to produce blood glucose meter panels that met the following requirements:
- Material: PC – It offers impact resistance, improved transparency, and enhanced compliance with ISO 10993 biocompatibility standards.
- Product Specifications: 20g per piece, 85mm x 70mm in size.
- Mold Configuration: A two-cavity mold is used (to balance production output and part quality; a four-cavity mold risks uneven cooling of thin-walled PC).
- Cycle Time: Targeted at approximately 30 seconds.
- Quality Standards: Dimensional tolerance of ±0.05mm, zero flash, and 100% traceability.
Preliminary trials with the customer revealed that the existing hydraulic injection molding machine had drawbacks: cycle times as long as 38 seconds, 1.5% of parts exhibited dimensional errors, and hydraulic system oil leaks posed a contamination risk.
Why Electric Injection Molding Machines Are More Suitable for Medical Injection Molding
Medical injection molding requires three essential qualities: precision, purity, and repeatability—recognizing the advantages of electric injection molding machines over hydraulic ones. First, hydraulic injection molding machines rely on oil pressure for accuracy, which fluctuates with temperature and wear, leading to inconsistent injection speed and pressure. Electric injection molding machines use servo motors on each axis (injection, clamping, and ejection) to achieve positioning accuracy up to ±0.1 mm, which allows them to meet the 0.05 mm tolerances required for blood glucose meter panels. Secondly, regarding purity, hydraulic systems pose the risk of oil leaks, which could contaminate medical-grade materials like PC. The oil-free design of all electric injection molding machines eliminates this risk.
Furthermore, electric injection molding machines maintain consistent cycle times and part quality over over 10,000 runs. In customer testing, blood glucose meter panels produced using electric injection molding machines achieved a repeatability of 99.8%, compared to only 97.2% for hydraulic machines. For medical manufacturers, this consistency means fewer rejected batches, faster FDA approval, and reduced waste.
Advantages of the TEll 160T Electric Injection Molding Machine
First, the 160-ton clamping force is sufficient to maintain a tight seal in a two-cavity mold during injection, preventing flash. Unlike lower-tonnage injection molding machines that struggle with PC’s high melt viscosity, the TEll 160t’s clamping system maintains stable pressure throughout the entire molding cycle. Secondly, regarding injection performance, the machine’s injection unit boasts a maximum speed of 500 mm/s, making it ideal for filling thin-walled panels without creating bubbles.
This precise control reduces fill time per cavity to 3 seconds, compared to 5 seconds for a hydraulic press. Thirdly, the cycle time for medical injection molding has been optimized. By streamlining each stage, the TEll 160t achieved a total cycle time of 29 seconds, one second less than the customer’s target: an injection and holding time of 10 seconds, a cooling time of 12 seconds (PC requires longer cooling time to avoid warping), and an ejection time of 7 seconds. This 29-second cycle equates to a production output of 1,240 parts per hour, exceeding the 947 parts per hour achieved by a hydraulic press.

How is medical-grade PC processed in medical injection molding?
Medical-grade PC is a challenging material for medical injection molding, requiring precise temperature control, uniform melt distribution, and zero contamination. The TEll 160t successfully handles these materials with its specialized features for medical injection molding. The machine’s barrel features five independent heating zones, each maintaining a temperature range of 300–320°C. This eliminates hot spots that can cause material degradation. Furthermore, the TEll 160t’s hopper is equipped with a dehumidifier and dust filter, which is also critical for PC. Customers report zero defects due to moisture absorption after switching to the TEll 160t, compared to a 0.8% defect rate on their previous machine. Third, sensors built into the machine track melt pressure in real time, alerting the operator through the control panel before abnormalities impact part quality. For the blood glucose meter panel, this means each part has consistent clarity and strength, meeting the customer’s drop test durability requirements.

Meeting Production Standards for Medical Device Products
Compliance is essential for medical injection molding. The TEll 160t injection molding machine streamlines regulatory compliance for blood glucose meter panel production in three key ways. First, the system logs data throughout the entire process, automatically recording every critical parameter for each part. This allows customers to retrieve records during audits quickly. Second, the system performs defect detection. An integrated camera inspects each blood glucose meter panel as it ejects, checking for dimensional errors, flash, or scratches. The system automatically sorts defective parts to reduce human error. Furthermore, the injection molding machine holds ISO certification. The TEll 160t complies with ISO 13485 (Quality Management for Medical Devices) and ISO 9001 standards, meaning customers can meet regulatory requirements without modifying the machine.
Delivering flawless blood glucose meter panel production
This case study demonstrates that the right electric injection molding machine can transform medical plastics production from a high-risk, inefficient process to a reliable and scalable one. For the customer, the TEll 160t achieved a cycle time of 29 seconds, a defect rate of 0.2%, and 100% compliance with FDA and ISO standards. For medical manufacturers producing similar precision parts, all-electric injection molding machines not only produce better parts but also reduce risk and cut costs.
TRENDING POSTS
- TOPSTAR Global Open Day 2025: Humanoid Robot Debuts, Pioneering a New Decade of Intelligent Manufacturing 2025/10/10
- Topstar Showcases TE II Electric Injection Molding Machines at InterPlas Thailand 2025 2025/10/10
- Topstar Expands Its Ecosystem Partnerships to Drive Smart Manufacturing Innovation 2025/10/10
- What factors can cause delays in the injection molding process of plastic molding machine? 2025/10/10
HOT TOPIC
- .ervo motor-driven linear robots
- •
- 1.0 guangdong topstar technology co. ltd
- 1.0 topstar china
- 1.0 topstar robot
- 11
- 160℃ mold temperature controller
- 170 ton injection molding machine
- 2
- 21
- 220-ton injection molding machine
- 23
- 260 ton injection molding machine
- 3 axis robot
- 3 axis robots
- 3 in 1 Compact Dehumidifying Dryer
- 3-axis robot
- 3-axis robots
- 39
- 41
- 460T injection molding machine
- 5-axis CNC machine
- 62
- 90 ton injection molding machine
- accuracy
- Air Chillers
- all electric injection molding machine
- all electric injection molding machines
- all-electric injection molding machine
- All-electric injection molding machines
- and overall production quality. Therefore
- AP-RubberPlas
- auto loader
- automated injection molding machine
- Automation changed engineering
- automation of injection molding robots
- automotive parts injection molding
- Auxiliary Equipment
- auxiliary machine
- Bench Injection Molding Machine
- cabinet dryer
- Cabinet dryer manufacturers
- Cabinet dryers
- chiller
- CNC Drilling Machine
- CNC Drilling Machines
- cnc engraving machine manufacturer
- cnc laser cutting machine manufacturer
- CNC machine
- CNC Machine Center
- CNC Machine for Sale
- CNC Machine Manufacturing
- CNC Machine Tool
- CNC machine tool product
- CNC Machining Center
- CNC wood carving machine
- Cooling system
- Cross-Walking Single Axis Servo Cylinder Robot
- Cross-Walking Single-Axis Servo Cylinder Robot
- Cross-Walking Three-Axis/Five-Axis Servo Driven Robot
- cross-walking three-axis/five-axis servo-driven robot
- Dehumidifier Dryer
- Dehumidifying Dryer
- delta parallel robot
- Desktop Injection Molding Machine
- Desktop injection molding machines
- Desktop Molding Machine
- desktop plastic injection machine
- Desktop Plastic Injection Molding Machine
- Digital Transformation
- direct clamp injection molding machine
- Direct clamp injection molding machines
- Dosing & mixing system
- Drilling Centers
- Drying and dehumidification system
- drying and dehumidifying equipment
- Drying and Dehumidifying System
- drying system
- effective and efficient. Cabinet dryers are also used in other industries where large quantities of material need to be dried
- efficient injection molding machine
- elbow hydraulic injection molding machines
- electric injection molding machine
- electric injection molding machines
- energy-efficient injection molding robot
- energy-efficient water chiller
- energy-efficient water chillers
- energy-saving injection molding machine
- etc. Among injection molding robots
- exhibition
- features of CNC machine
- Feeding And Conveying System
- Five Axis Machine Center
- Flexible Production Line
- Fully automatic injection molding machine
- Gathering Topstar
- giant injection molding machine
- GMU-600 5-Axis Machining Center
- Granulating & Recycling System
- granulator machine
- gravimetric blender
- Heavy duty injection molding machine
- High-precision electric molding machines
- high-precision plastic molding machines
- high-speed all electric injection molding machine
- high-speed electric injection molding machine
- High-Speed Packaging Injection Molding
- Honeycomb rotor dehumidifier
- Hopper Dryers
- horizontal injection molding machine
- Horizontal Injection Molding Machines
- Horizontal Injection Moulding Machine
- Horizontal Mixer manufacturer
- How The CNC Machine Works
- hybrid injection molding machine
- hydraulic injection molding machine
- Hydraulic Injection Molding Machines
- in this article
- Industrial AI
- Industrial Automation
- Industrial robot
- Industrial Robot Chinese brand
- industrial robot parts
- industrial robot supplier
- Industrial robots
- Industry Chain
- Injection Manipulator
- injection manipulator robot
- injection mold machines
- Injection molding
- Injection molding automation
- Injection Molding Automation Solution
- injection molding dryer
- Injection molding equipment
- injection molding hopper dryer
- Injection molding machine
- injection molding machine brand
- Injection Molding Machine Factory
- Injection Molding Machine Manufacture
- Injection molding machine manufacturer
- injection molding machine manufacturers
- Injection molding machine procurement
- injection molding machine robotic arm
- injection molding machine with a robot
- Injection molding machines
- injection molding material dehumidifying
- injection molding plant
- injection molding process
- Injection Molding Robot
- injection molding robot arm
- Injection molding robot automation
- Injection molding robotic arm
- injection molding robots
- Injection moulding machine
- injection moulding machines
- Injection Moulding Robots
- Injection Robot
- Injection robot arm
- Injection robot manufacturer
- Injection robot wholesale
- injection robots
- Intelligent Factory
- intelligent injection molding machines
- Intelligent Manufacturing
- intelligent mold temperature
- intelligent mold temperature controller
- Intelligent mould temperature controller
- InterPlas Thailand 2025
- Introducing Injection Robot
- It is the best choice for drying large quantities of material at once. Cabinetmakers use these machines because they are fast
- Large flow water type mold temperature controller
- large injection molding machine
- large injection molding machines
- Learn what industrial automation and robotics is
- linear robot
- linear robots
- low speed sound-proof granulator
- machine plastic molding
- make sure to add some! Improvements (2) Keyphrase in introduction: Your keyphrase or its synonyms appear in the first paragraph of the copy
- manipulator machine
- manufacturing
- Manufacturing Innovation
- medical grade injection molding machines
- Medical Injection Molding
- medical injection molding machine
- medical injection molding machines
- micro injection molding machine
- middle speed granulator
- Mini CNC machine manufacturers.
- mobile cover making machine
- Mold Temperature Control System
- mold temperature controller
- mold temperature controllers
- molding machine
- molding material Dehumidifying System
- mould temperature control system
- mould temperature controller
- mould temperature controllers
- New electric injection molding machine
- nitrogen dryer manufacturer
- nitrogen dryer system manufacturer
- Oil type mold temperature controller
- Oil type mold temperature controllers
- open day
- optical component injection molding
- Outbound links: No outbound links appear in this page. Add some! Images: No images appear on this page. Add some! Internal links: No internal links appear in this page
- packaging injection molding
- Packaging Solutions
- PET Preform injection molding
- phone case maker machine
- phone case making machine
- phone cover making machine
- PID Control Mold Temperature Controller
- plastic auto loader
- plastic bottle making machine
- plastic bottle manufacturing
- plastic bucket making machine
- plastic bucket manufacturing
- Plastic chair making machine
- plastic dryer for injection molding
- plastic forming equipment
- Plastic Granulators
- plastic hopper dryer
- plastic injection machine
- plastic injection machines
- plastic injection molding
- Plastic injection molding equipment
- Plastic injection molding machine
- Plastic Injection Molding Machines
- plastic injection moulding machine
- plastic injection moulding machines
- plastic injection robot
- plastic molding
- Plastic Molding Industry
- Plastic Molding machine
- plastic molding machine 1
- Plastic Molding Machines
- plastic molding press
- plastic moulding machine
- plastic phone case making machine
- plastic-molding machine
- powerful granulator
- Powerful Type Sound-Proof Granulator
- precision injection molding
- precision injection molding machines
- production of plastic seats
- pure water mould temperature controller
- Robot injection molding
- robot injection molding machine
- robot manufacturing companies
- Robotic arm for injection molding machine
- robotic injection molding machines
- robotics in injection molding
- SCARA robot
- SCARA robots
- Screw dosers
- Service-oriented manufacturing
- Servo Cylinder Robot
- servo driven robot
- Servo Driven Robots
- servo injection molding machine
- servo injection robots
- servo motor-driven linear robots
- servo-driven 3-axis robot
- Servo-driven injection molding machine
- Servo-Driven Robot
- Setup of injection machine
- Silicone Injection Molding Machine
- six-axis industrial robot
- Smart Manufacturing
- soundproof granulator
- Stainless Hopper Dryer
- Stainless Hopper Dryers
- star club
- swing arm robot
- take-out robot
- take-out robots
- Thailand 4.0
- the choice between servo-driven robots and hydraulic robots will have a certain impact on efficiency
- the most popular injection molding machine
- the type of injection molding robot
- TIC2000 Control System
- TMII injection molding machine
- toggle clamp injection molding machine
- Toggle Hydraulic Injection Molding Machines
- toggle injection molding machine
- Top 10 brands of injection robots
- Topstar
- Topstar Electric Injection Molding Machine InterPlas Thailand 2025 Smart Manufacturing Thailand 4.0
- Topstar Engineering
- Topstar Industrial Robots
- Topstar injection molding intelligent
- Topstar Scara Robots
- Useful Injection molding machine
- Vertical machining centers
- volumetric type blender
- water chiller
- water chillers
- water distributor
- water type mold temperature controller
- Water Type MoldTemperature Controller
- Water-Type Mould Temperature Controllers
- We often face choices when performing injection molding. We will choose the type of injection molding machine
- wholesale of injection molding machines
- x carve CNC
- 热门查询 点击次数 展示 排名 topstar
